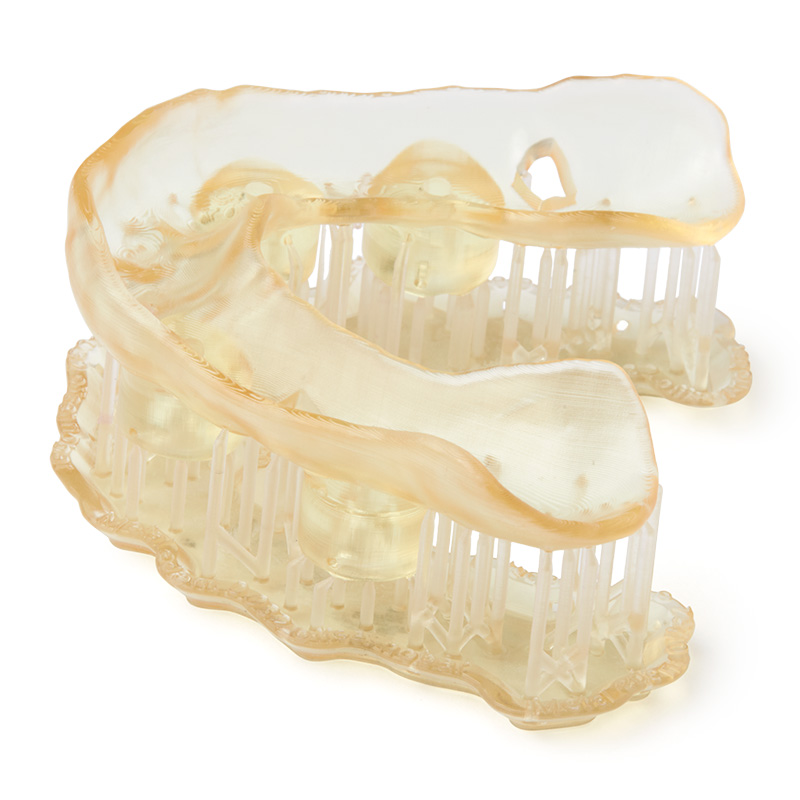
Read the Formlabs Application Guide for the complete digital workflow and best practices for creating 3D-printed surgical manuals.
Certifications
Formlabs Surgical Guide resin is manufactured according to EN ISO 13485:2016 and EN ISO 14971:2012 for medical applications. These certifications ensure the necessary quality control and risk management.
Formlabs Surgical Guide has the following biocompatibility certificates:
- EN ISO 10993-5: Non-cytotoxic
- EN ISO 10993-10: Non-irritating
- EN ISO 10993-10: Non-sensitizing
Disinfection
Parts printed with this resin can be washed/disinfected in 70% isopropyl alcohol. We recommend exposing parts to isopropyl alcohol for no longer than 5 minutes.
Cleaning and sterilization
- It is possible to clean parts with the main medical sterilization methods:
- Autoclave: 20 minutes at 134°C and 30 minutes at 121°C
- Ethylene oxide: immersion in 100% Ethylene oxide 55°C for up to 3 hours
- E-beam: 35 kGy E-beam radiation
- Gamma: 29.4 – 31.2 kGy gamma radiate
Formlabs Biomed for the Form 3B and 3BL
This resin is also available for the previous generation Formlabs SLA 3D printers: Formlabs Surgical Guide (Form 3)